The law of conservation of mass states that in a chemical reaction mass is neither created nor destroyed. For example, the carbon atom in coal becomes carbon dioxide when it is burned. The carbon atom changes from a solid structure to a gas but its mass does not change.
Law of conservation of mass – Stock Image – A500/0828 – Science Photo Library
Oct 19, 2023The same amount of matter exists before and after the change—none is created or destroyed. This concept is called the Law of Conservation of Mass. In a physical change, a substance’s physical properties may change, but its chemical makeup does not. Water, for example, is made up of two hydrogen atoms and one oxygen atom.

Source Image: m.youtube.com
Download Image
Law of Conservation of Mass. The law of conservation of mass was created in 1789 by a French chemist, Antoine Lavoisier. The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. For example, when wood burns, the mass of the soot, ashes, and gases equals the original mass of the charcoal and the oxygen when it first reacted.

Source Image: m.youtube.com
Download Image
31+ Example Of Law Of Conservation Of Mass: Detailed Explanations – YouTube
(Jan. 11, 2024) conservation of mass, principle that the mass of an object or collection of objects never changes, no matter how the constituent parts rearrange themselves. Mass has been viewed in physics in two compatible ways.

Source Image: shutterstock.com
Download Image
What Does The Law Of Conservation Of Mass State
(Jan. 11, 2024) conservation of mass, principle that the mass of an object or collection of objects never changes, no matter how the constituent parts rearrange themselves. Mass has been viewed in physics in two compatible ways.
Apr 17, 2023LICENSED UNDER. 3.6: Conservation of Mass is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. The law of conservation of mass states that matter can not be created or destroyed in a chemical reaction. So the mass of all of the products formed equals the mass of all of the reactants that were ….
4+ Thousand Conservation Mass Royalty-Free Images, Stock Photos & Pictures | Shutterstock
The law of conservation of mass is that, in a closed or isolated system, matter cannot be created or destroyed. It can change forms but is conserved. Law of Conservation of Mass in Chemistry
7+ Hundred Conservation Mass Royalty-Free Images, Stock Photos & Pictures | Shutterstock

Source Image: shutterstock.com
Download Image
Law Of Conservation Of Mass Images – Browse 1,174 Stock Photos, Vectors, and Video | Adobe Stock
The law of conservation of mass is that, in a closed or isolated system, matter cannot be created or destroyed. It can change forms but is conserved. Law of Conservation of Mass in Chemistry

Source Image: stock.adobe.com
Download Image
Law of conservation of mass – Stock Image – A500/0828 – Science Photo Library
The law of conservation of mass states that in a chemical reaction mass is neither created nor destroyed. For example, the carbon atom in coal becomes carbon dioxide when it is burned. The carbon atom changes from a solid structure to a gas but its mass does not change.

Source Image: sciencephoto.com
Download Image
31+ Example Of Law Of Conservation Of Mass: Detailed Explanations – YouTube
Law of Conservation of Mass. The law of conservation of mass was created in 1789 by a French chemist, Antoine Lavoisier. The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. For example, when wood burns, the mass of the soot, ashes, and gases equals the original mass of the charcoal and the oxygen when it first reacted.
Source Image: youtube.com
Download Image
Pin by Peggy Kennedy on Chemistry | Conservation of mass, Science homework, Chemistry
In physics and chemistry, the law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as the system’s mass cannot change, so the quantity can neither be added nor be removed.

Source Image: pinterest.com
Download Image
Law of Conservation of Mass | Don’t Memorise – YouTube
(Jan. 11, 2024) conservation of mass, principle that the mass of an object or collection of objects never changes, no matter how the constituent parts rearrange themselves. Mass has been viewed in physics in two compatible ways.

Source Image: m.youtube.com
Download Image
Law of Conservation of Mass Vector Illustration. Labeled Educational Scheme Stock Vector – Illustration of dioxide, angular: 168491146
Apr 17, 2023LICENSED UNDER. 3.6: Conservation of Mass is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. The law of conservation of mass states that matter can not be created or destroyed in a chemical reaction. So the mass of all of the products formed equals the mass of all of the reactants that were ….
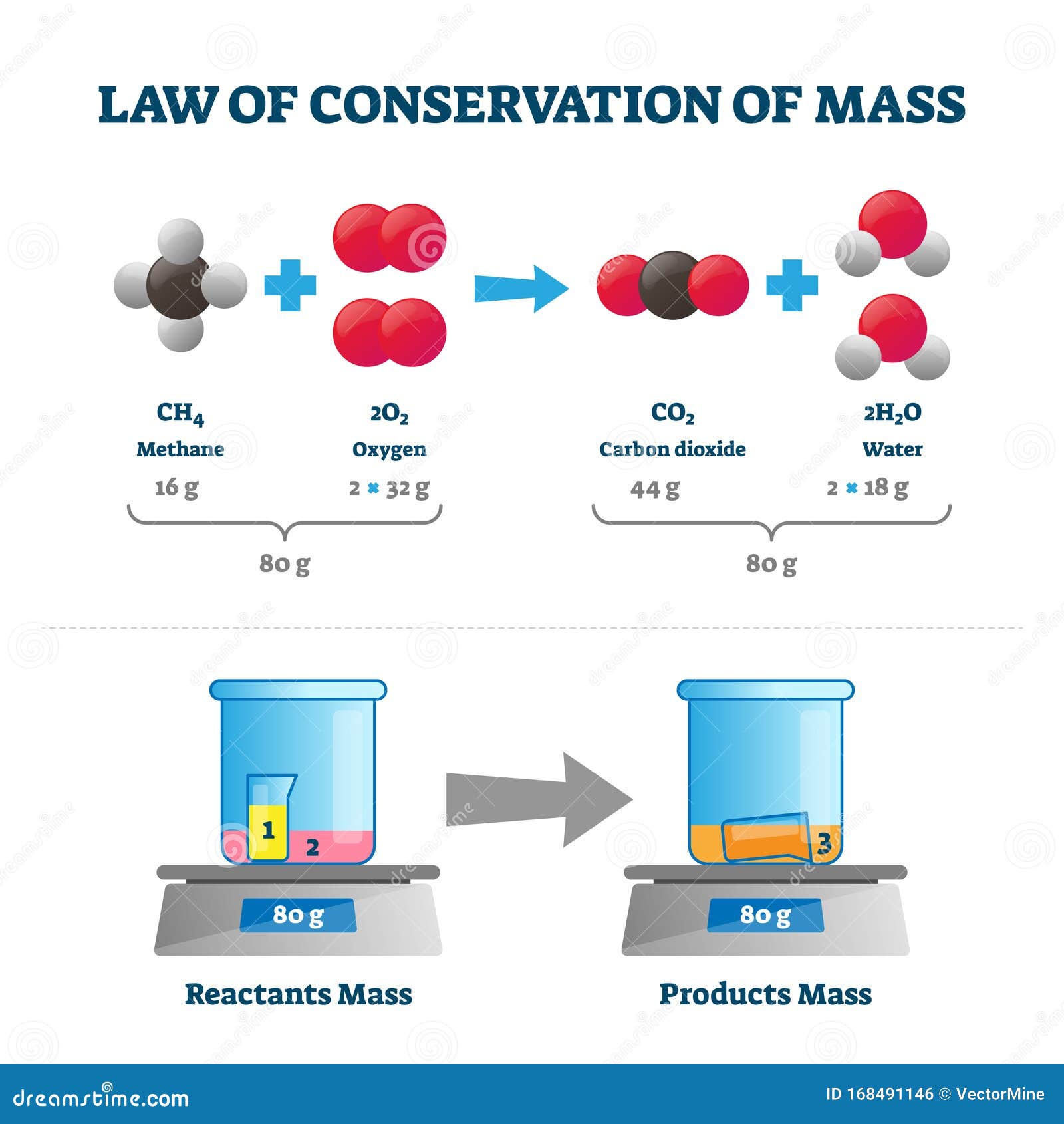
Source Image: dreamstime.com
Download Image
Law Of Conservation Of Mass Images – Browse 1,174 Stock Photos, Vectors, and Video | Adobe Stock
Law of Conservation of Mass Vector Illustration. Labeled Educational Scheme Stock Vector – Illustration of dioxide, angular: 168491146
Oct 19, 2023The same amount of matter exists before and after the change—none is created or destroyed. This concept is called the Law of Conservation of Mass. In a physical change, a substance’s physical properties may change, but its chemical makeup does not. Water, for example, is made up of two hydrogen atoms and one oxygen atom.
31+ Example Of Law Of Conservation Of Mass: Detailed Explanations – YouTube Law of Conservation of Mass | Don’t Memorise – YouTube
In physics and chemistry, the law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as the system’s mass cannot change, so the quantity can neither be added nor be removed.